We are ROTOP Pharmaka GmbH, a leading pharmaceutical manufacturer in Germany, headquartered in Dresden-Rossendorf. For more than 25 years, we have developed, manufactured, and supplied cGMP-compliant radiopharmaceuticals for diagnostics and therapy in nuclear medicine and molecular imaging – distributed to more than 40 countries worldwide.
The ROTOP brand stands for quality, reliability, and pharmaceutical expertise. As a radiopharmaceutical manufacturer, we focus on close customer relationships, collaborative partnerships, and tailored radiopharmaceutical solutions for hospitals, clinics, and research partners. Our mission: advancing the medicine of tomorrow – developed and manufactured with responsibility.
ROTOP Pharmaka stands for integrity, expertise, and commitment – from Dresden to the future of nuclear medicine.
Driving Theranostic Innovation for a brighter future.
Our Vision
Our vision is to become a full-service provider in the field of radiopharmacy. Through innovative radionuclide theranostics, we aim to drive medical progress and contribute to a world where serious diseases can be detected early and treated effectively. We are committed to close collaboration with medical professionals and partners to push the boundaries of what’s possible—improving health and quality of life for people around the world.
Our Mission
For Patients: Our theranostics provide patients with clarity and sustainably improve their quality of life.
For Customers: We offer our customers tailored services covering the development, manufacturing, and distribution of theranostics. We are passionate about understanding their individual needs and requirements, and we provide comprehensive support throughout the entire product lifecycle.
For Employees: We are a team defined by integrity, knowledge, and commitment. We foster motivation, initiative, and a thirst for knowledge—and offer development opportunities across all areas.
Our Values
Our values are the foundation of our work and serve as the guiding principles for our actions and decisions.
Integrity
We are committed to fairness and respectful collaboration. Our work is built on transparency and integrity.
Knowledge
We combine deep expertise, years of experience, and innovative approaches. Our dedicated team of experts helps ensure the highest quality standards and delivers outstanding products and services to our customers.
Commitment
We are committed to driving innovation and progress. Through our dedication to nuclear medicine, we actively contribute to the advancement of the field. We continuously strive to develop customized solutions for our customers while fostering a work environment that inspires and motivates our employees to give their best. Beyond our core business, we are actively engaged in social and community matters—working to make a positive impact and drive sustainable change.
OUR KNOW-HOW
More than 25 years of experience in radiopharmacy
Our company traces its origins to the former Central Institute for Nuclear Research in Rossendorf, where the production of radioactive materials began in 1958. Since the foundation of ROTOP in 2000, we have evolved into a modern, internationally active radiopharmaceutical manufacturer. The distinctive ROTOP logo represents precision, reliability, and the highest quality standards in radiopharmacy.
Over the years, we have continuously expanded our portfolio: from early Cold Kits and radionuclide generators to the CDMO development of Ga-68-PSMA and our first non-generic product, Tektrotyd. With the launch of radioactive cGMP manufacturing in 2020 and the introduction of our CDMO services in 2021, we strengthened our integrated radiopharmaceutical value chain. Since 2025, we have expanded our capacity for clinical trial materials (alpha, beta, and gamma emitters), and beginning in 2026, we will further grow our cleanroom facilities to increase radiopharmaceutical production through 2028.
Why choose ROTOP?
SERVICES & CAPABILITIES
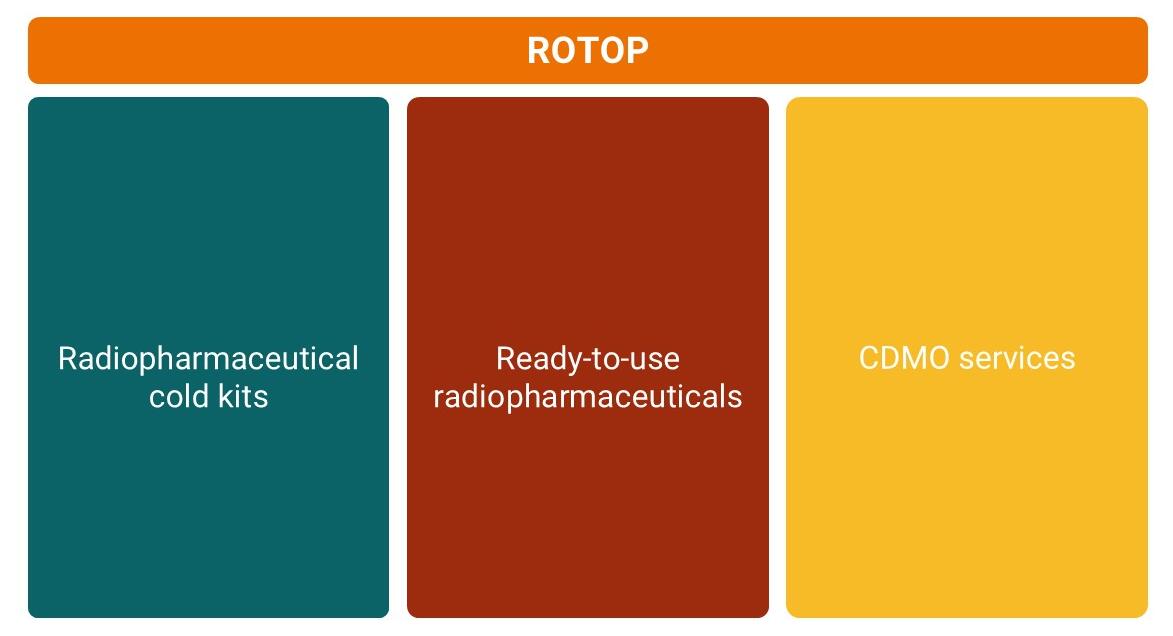
Our business units
With more than 25 years of experience in the development and manufacturing of radiopharmaceuticals, we support the entire lifecycle of our products — from research and analytical development to cold kit formulation, manufacturing, and regulatory approval in Germany and the European Union.
Our expertise includes sterile lyophilisates, injectable solutions, and radionuclide generators for clinical use. Through our CDMO Services (Contract Development and Manufacturing Organization), we support partners in developing, registering, and manufacturing clinical trial materials and market-ready radiopharmaceutical kits — from the initial concept to the fully commercialized product.
GMP-COMPLIANT PHARMACEUTICAL MANUFACTURING FOR NUCLEAR MEDICINE
Radiopharmaceutical cold kits
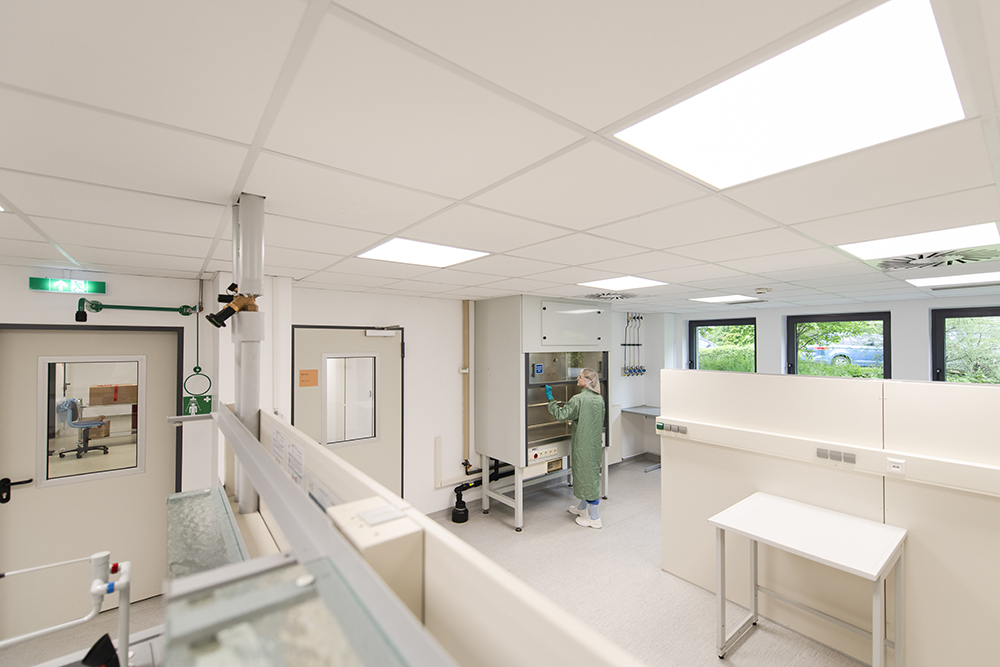
In our own cleanrooms on the campus of Helmholtz-Zentrum Dresden-Rossendorf, we produce sterile radiopharmaceutical cold kits (Tc-99m cold kits) for nuclear medicine diagnostics. They serve as a reliable basis for radioactive labeling in clinics and medical practices worldwide – for example in functional disorders, oncological indications, and modern imaging procedures.
Our GMP-compliant manufacturing includes formulation development, lyophilization, filling, and final quality control. Strict quality testing, state-of-the-art equipment, and fully GxP-compliant processes ensure maximum safety, reproducibility, and international quality standards for efficient and patient-focused diagnostics.
What defines us as a radiopharmaceutical manufacturer
GMP-COMPLIANT PRODUCTION OF READY-TO-USE RADIOPHARMACEUTICALS FOR NUCLEAR MEDICINE
Ready-to-use radiopharmaceuticals
At our ROTOP Radiopharmacy, we produce ready-to-use radiopharmaceuticals for nuclear medicine diagnostics. The modern facility started operations in 2020 and features three GMP-compliant hot cells for processing high activities of iodine-123.
Because all steps—from radionuclide production at our on-site cyclotron to manufacturing, automated filling, and packaging—are consolidated in one location, we ensure maximum supply security and the highest quality standards.
Our patient doses are produced individually and shipped across Europe. Semi-automated packaging in eco-friendly Type-A containers, as well as free return of lead containers, are part of our service. Optimized logistics enable reliable delivery within 48 hours.
Insights into the work of ROTOP Radiopharmacy
YOUR TRUSTED CDMO PARTNER FOR RADIOPHARMACEUTICALS
CDMO services
As a full-service CDMO, ROTOP supports your radiopharmaceutical projects from early-stage development to commercial supply – all under one roof. Our expertise covers precursor and API synthesis, development of lyophilized cold kits, process scale-up, clinical and commercial GMP manufacturing, integrated quality control, and regulatory support for the EU, USA, and RoW.
Our state-of-the-art facilities in Dresden-Rossendorf include hot cells, modular cleanrooms, precursor laboratories, and in-house QC labs that ensure maximum product safety, reliability, and reproducibility. We support a wide range of radionuclides and deliver efficiently to clinics and partners worldwide through just-in-time logistics. With more than 18,800 sq ft of laboratory and cleanroom space and the capacity to produce up to 5,000 vials per batch, ROTOP offers a true one-stop shop for complete radiopharmaceutical development and manufacturing.
Our management team

Jens Junker
CEO

Dr. Antje Sterger
COO

Dr. Thomas Gottlieb
CBO

Hendrikje Heinrich
CTO

Andrew Varghese
President, ROTOP US & CDMO Services

Dr. Eik Schiller
CSO
Quality, Safety & Responsibility
Our GMP-certified pharmaceutical manufacturing in Dresden ensures the highest standards of quality and safety. Active substances, cold kits and liquid solutions are produced strictly in accordance with Good Manufacturing Practice (GMP). A comprehensive and independent quality management system oversees and controls every step – from contract manufacturing and quality control to the international distribution of radiopharmaceuticals.
This consistent commitment to quality is more than a requirement – it reflects our responsibility toward patients, partners and the scientific community.
ROTOP stands for integrity, expertise and commitment – from Dresden, driving the future of medicine.
At ROTOP Pharmaka, patient safety always comes first. At the same time, we recognize that as our radiopharmaceutical company grows, so does our responsibility for sustainability and environmental protection. To meet this ethical obligation, we established an interdisciplinary project team in early 2022 dedicated to sustainability in radiopharmacutical manufacturing and to developing a comprehensive sustainability strategy for ROTOP.
Our team
At ROTOP, our team is the base of our success. Our heads of department combine many years of experience with deep expertise in research, regulatory affairs, manufacturing and the distribution of radiopharmaceuticals.
We value diversity and collaboration: different perspectives and specialized knowledge enable us to develop innovative solutions and meet the high standards of the radiopharmaceutical industry. Our company values – integrity, expertise and commitment – shape our daily work and the way we collaborate, from the laboratory to administration.
Together, we advance nuclear medicine, develop modern diagnostics and ensure the quality of our products for patients around the world.
Shape the future of Radiopharmaceuticals with us
Your career at ROTOP
We offer diverse career paths in manufacturing, research, administration, and sales—empowering our employees to contribute to the future of diagnostic imaging and theranostics. Become part of a dedicated team driving medical innovation worldwide.