We’re Here for You
As a GMP-certified manufacturer of radiopharmaceuticals, we support hospitals, clinics, research institutions, and industry partners across the globe. If you have questions about our products, Tc-99m cold kits, ready-to-use radiopharmaceuticals, our CDMO services, or our company, we are happy to assist you.
General contact
For general inquiries, please reach out to:
ROTOP Pharmaka GmbH
Bautzner Landstrasse 400
01328 Dresden
GERMANY
Email: info@rotop-pharmaka.com
Phone: +49 351 26310 100
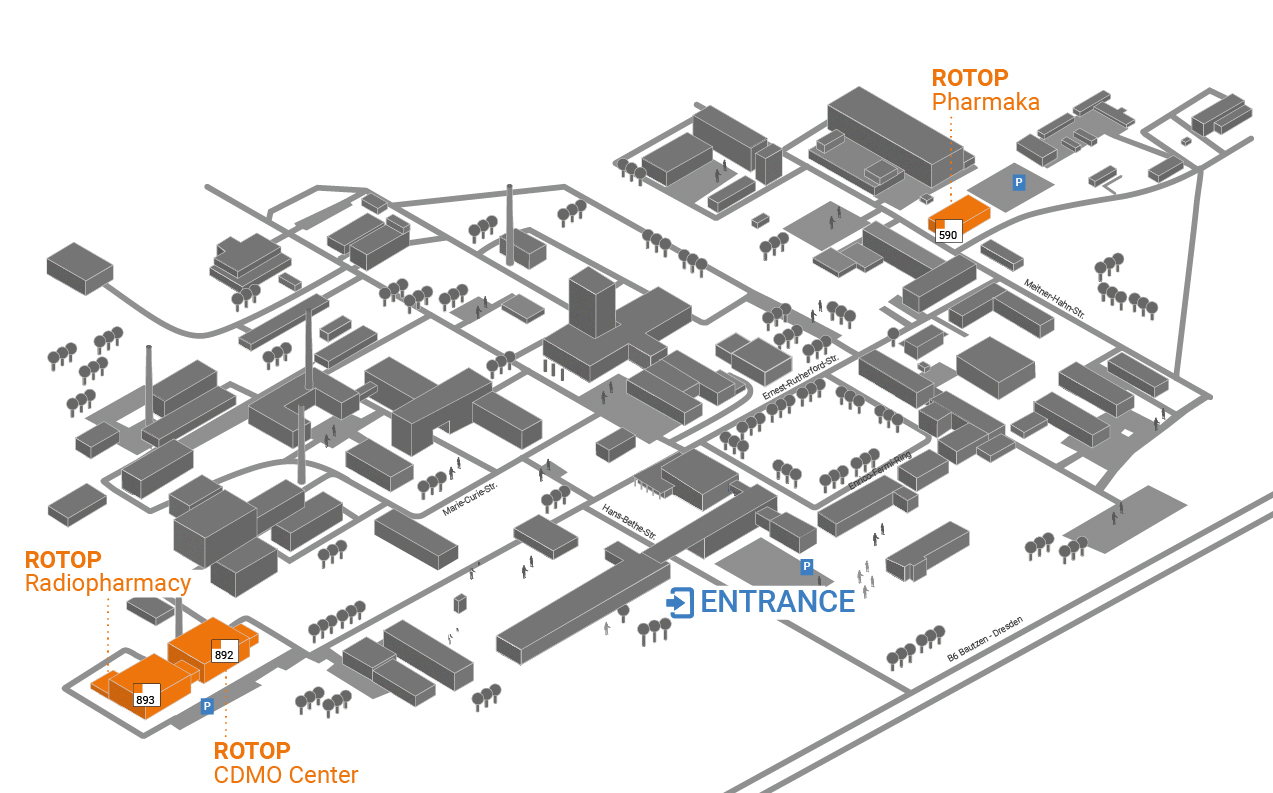
Our headquarters is located on the campus of the Helmholtz-Zentrum Dresden-Rossendorf.
Please note that visitor registration at the main gate is required to access the campus.
Plan an additional 5–10 minutes for registration during each visit, and remember to bring your ID card.
GETTING HERE
Directions
Our site in Dresden-Rossendorf is the center of our GMP manufacturing, pharmaceutical development and contract manufacturing activities. Easily accessible from Dresden, the Lusatia region and the surrounding area. On-site visitor parking available.
Note: The map is an embedding of Google Maps. Our company assumes no liability for the data handling and processing of your personal data by Google. The Google LCC Terms of Use and Privacy Policy apply.
PRODUCT INQUIRIES & ORDERS
Our customer service
Global
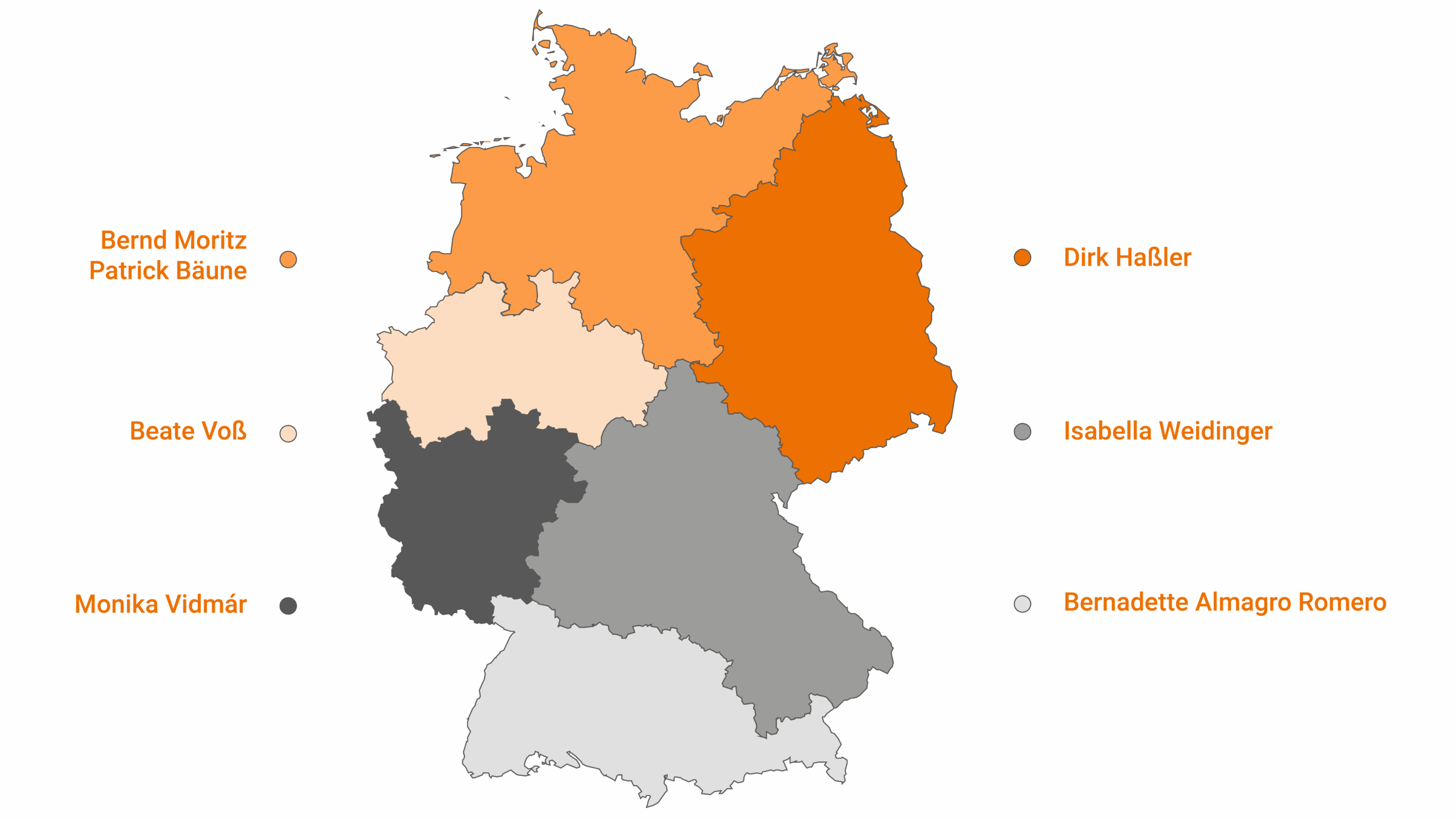
Germany, Austria, Switzerland
Phone: +49 351 26 310 210
E-mail: kundenservice@rotop-pharmaka.de
Contact hours:
monday – thursday: 7 a.m. – 4.30 p.m.
friday: 7 a.m. – 3.30 p.m.
ON-SITE SUPPORT
Our field service
Our field service team provides direct on-site support in Germany and Austria. You will receive expert guidance on our GMP-compliant 99mTc Cold Kits, ready-to-use radiopharmaceuticals, and radionuclide generators – from product specifications and usage instructions to delivery processes and quality control training.
Through close collaboration with physicians, MTRAs, and pharmacies, we ensure that your requirements are met quickly, reliably, and individually.
If you would like a personal appointment or have any questions about our products, our field service team is always happy to assist you.

DRUG SAFETY
Pharmacovigilance
By reporting adverse drug reactions (ADRs), including suspected cases, you help provide more information on the safety of this medicinal product. Please contact our Pharmacovigilance department: pharmacovigilance@rotop-pharmaka.de
CONTRACT DEVELOPMENT
CDMO Project Inquiries
For collaborations in CDMO (Contract Development and Manufacturing), including precursor synthesis, kit development, GMP manufacturing, or support with regulatory processes for the EU / USA / RoW, please contact the experts in our CDMO team: cdmo@rotop-pharmaka.com

MEDIA & SPRONSORING
Marketing
For media inquiries, press information, sponsorship requests, statements, or image material, please contact our Marketing department: marketing@rotop-pharmaka.de
Shape the future of Radiopharmaceuticals with us
Your career at ROTOP
We offer diverse career paths in manufacturing, research, administration, and sales—empowering our employees to contribute to the future of diagnostic imaging and theranostics. Become part of a dedicated team driving medical innovation worldwide.